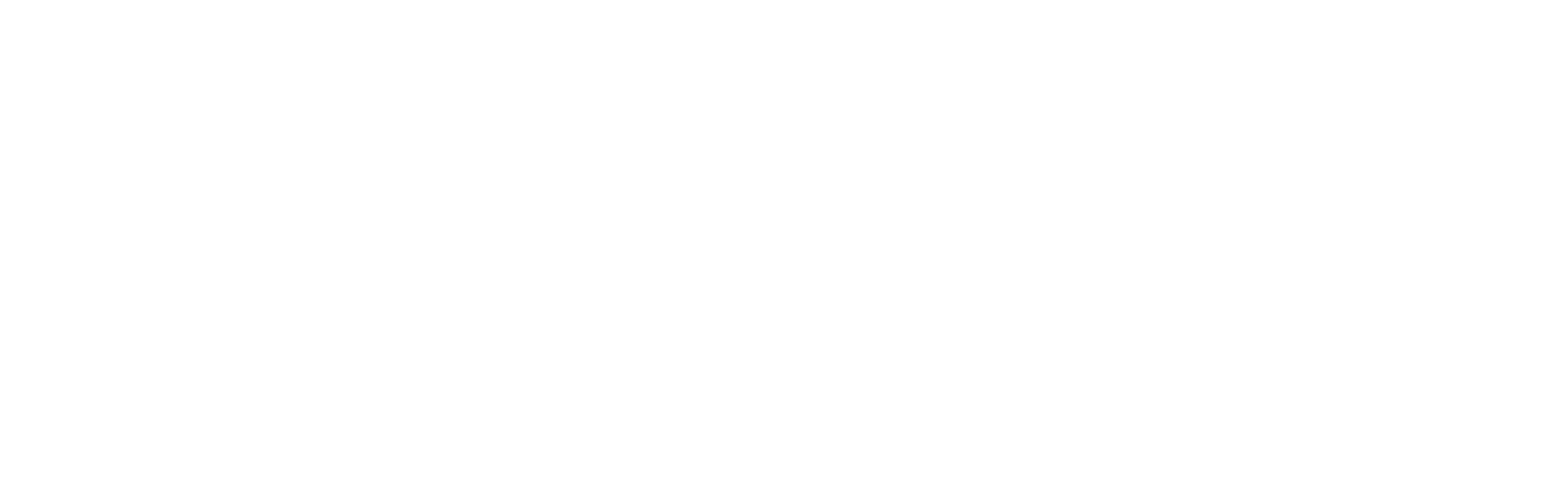Title
Clonal hematopoiesis of indeterminate potential is associated with acute kidney injury

Dr. Kestenbaum is a professor of medicine, nephrologist, and investigator at the KRI. His research interests include the evaluation of novel kidney functions, clinical assessment of acute kidney injury, and the early pathogenesis of diabetic kidney disease.
About
HIP - or clonal hematopoiesis of indeterminate potential - is a newly recognized condition characterized by acquired mutations in bone marrow stem cells. These specific mutations produce clonal populations of white blood cells that predispose to not only cancer, but also to several chronic disorders linked with dysregulated inflammation, including cardiovascular, pulmonary, and liver diseases. CHIP affects more than 10% of people over the age of 60.
Acute kidney injury describes a sudden loss of kidney function that occurs over hours-days. AKI complicates up to 20% of hospitalizations and is associated with substantial healthcare costs and patient mortality. AKI is perpetuated by diffuse inflammation within the kidneys and eventual scarring.
We hypothesized that CHIP would be associated with greater risks of AKI. To test this hypothesis, we determined associations in more than 400,000 individuals from the UK Biobank (UKB); Atherosclerosis Risk in Communities Cohort; and the Cardiovascular Health Study, and we corroborated our results in an animal model of CHIP.
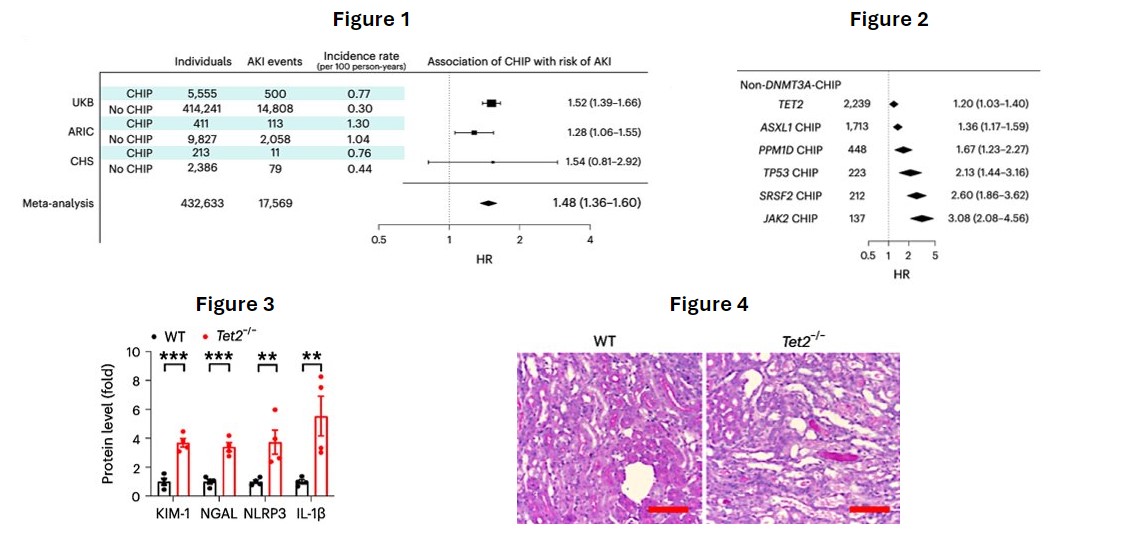
A total of 15,736 incident AKI events occurred over follow-up (3.1 events per 1,000 person-years). The presence of non-DNMT3A-CHIP was associated with an estimated 48% greater incidence of AKI (Figure 1). Mutations in CHIP driver genes JAKS2, SRSF2, and TP53 were associated with the greatest risks of AKI (Figure 2). In a mouse model of CHIP that recapitulated normal bone marrow with 20% cells carrying the CHIP Tet2−/− mutation, an exaggerated kidney pattern of injury was observed, with higher kidney concentrations of injury markers and more pronounced histologic evidence of tubular injury.
Author Information
Caitlyn Vlasschaert, Cassianne Robinson-Cohen, Jianchun Chen, Elvis Akwo, Alyssa C. Parker, Samuel A. Silver, Pavan K. Bhatraju, Hannah Poisner, Shirong Cao, Ming Jiang, Yinqiu Wang, Aolei Niu, Edward Siew, Joseph C. Van Amburg, Holly J. Kramer, Anna Kottgen, Nora Franceschini, Bruce M. Psaty, Russell P. Tracy, Alvaro Alonso, Dan E. Arking, Josef Coresh, Christie M. Ballantyne, Eric Boerwinkle, Morgan Grams, Ming-Zhi Zhang, Bryan Kestenbaum, Matthew B. Lanktree, Michael J. Rauh, Raymond C. Harris Jr, & Alexander G. Bick.
Publication
Nature Medicine
Volume 30 | March 2024 | 810–817