MENTOR: Membranous Nephropathy Trial Of Rituximab
Investigator

What is the MENTOR Study?
This is a multicenter, prospective, randomized, controlled Phase III trial. This study looks into determining whether Rituximab is non-inferior to Cyclosporine in inducing long-term remission of proteinuria in patients with Idiopathic Membranous Nephropathy.
Results
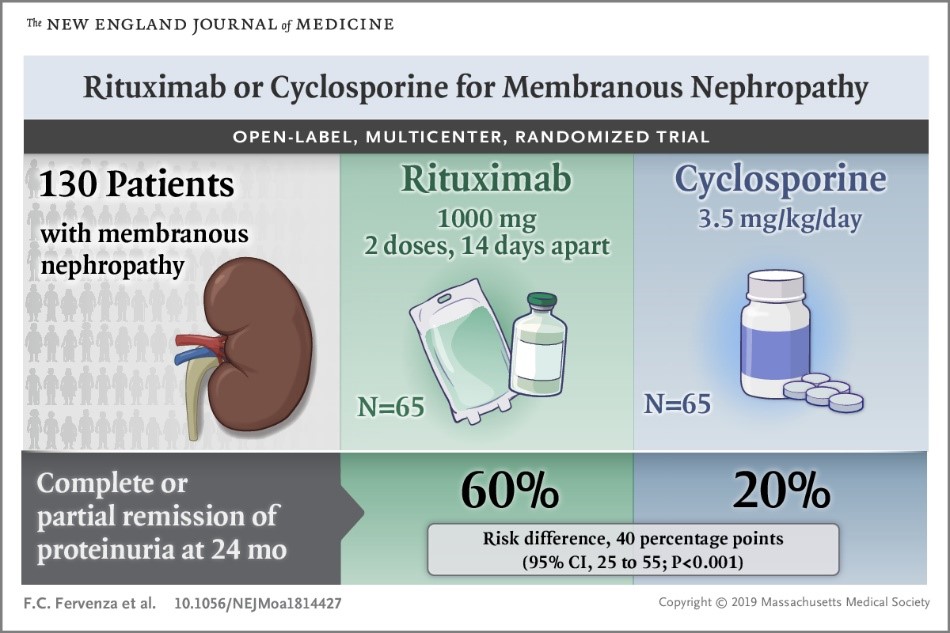
A total of 130 patients underwent randomization. At 12 months, 39 of 65 patients (60%) in the rituximab group and 34 of 65 (52%) in the cyclosporine group had a complete or partial remission. At 24 months, 39 patients (60%) in the rituximab group and 13 (20%) in the cyclosporine group had a complete or partial remission. Among patients in remission who tested positive for anti-phospholipase A2 receptor (PLA2R) antibodies, the decline in autoantibodies to anti-PLA2R was faster and of greater magnitude and duration in the rituximab group than in the cyclosporine group. Rituximab was non-inferior to cyclosporine in inducing complete or partial remission of proteinuria at 12 months and was superior in maintaining proteinuria remission up to 24 months.
Findings
Rituximab’s performance was equivalent to Cyclosporine at 12 months and superior at 24 months. Both drugs may be used to treat or prevent the progression of abnormal quantities of protein in the urine but this study showed that Rituximab may be the more effective option in the longer term.