This is unpublished
BESTOW
Active Studies
Transplantation
Pinned
Principal Investigator
Nicolae Leca, MD
Funding Agency
Sponsor: Eledon Pharmaceuticals
Status
Active
Enrollment closed
Investigator

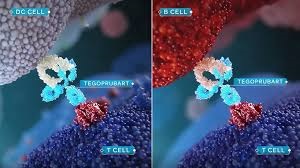
What is the Bestow Study?
A Phase 2, Multicenter, Randomized, Open-Label Study to Evaluate the Safety and Efficacy of Tegoprubart in Patients Undergoing Kidney Transplantation. -- To assess the graft function at 12 months post-transplant in tegoprubart participants compared to tacrolimus treated participants.
What are the criteria for the study?
Male or female greater than or equal to 18 years of age; recipient of first kidney transplant from living or deceased donor.
WHO DO I CONTACT FOR MORE INFORMATION?
Research Coordinator
Belinda Pan
E: bcpan@uw.edu