TITLE
Interferon-γ induces combined pyroptotic angiopathy and APOL1 expression in human kidney disease
First Author:

A postdoctoral research fellow at the KRI Benjamin is interested in studying the underlying mechanisms of how chronic inflammation contributes to kidney disease by using advanced in vitro models compared to clinical data. He is mentored by Benjamin Freedman, PhD.
About
Chronic inflammation plays a key role in various diseases, including those affecting the kidneys. One of the body's natural responses to pathogens involves interferons (IFNs), which are proteins that manage inflammation by turning on specific genes. High levels of interferons are linked to kidney disease, such as lupus nephritis and COVID- and HIV-associated nephropathy. Interferons are also known to increase the production of a protein called APOL1. Genetic variants of APOL1 offer protection against sleeping sickness when present in a single copy, but may also cause kidney disease in some people. The relationship between interferons and kidney disease is complicated, and it's unclear whether interferons directly contribute to kidney damage. In this study, we investigated how one type of interferon (IFN-γ) affects kidney-like structures grown in the lab called organoids , as well as endothelial cells from patient biopsies, to gain a better understanding of interferons’ broader impact on kidney health beyond APOL1 upregulation.
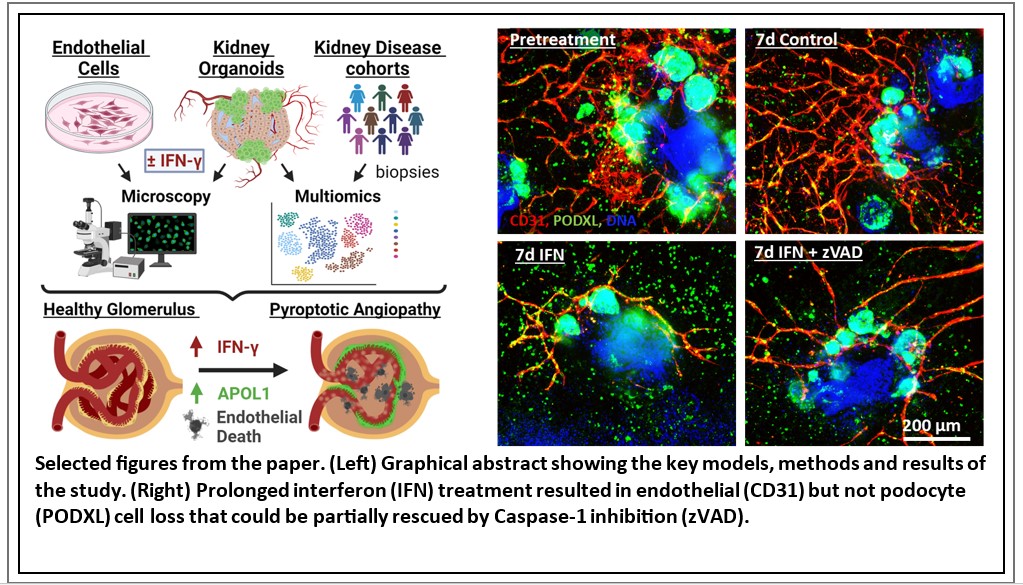
In this study we demonstrated that IFN-γ induces pyroptosis, a form of programmed cell death, in endothelial cells concurrently with APOL1 expression (Figure, left side) using human kidney organoids. Using this in vitro system we saw a dramatic loss of endothelial networks with prolonged IFN-γ exposure, which could be partially rescued by inhibiting caspase signaling with the pharmacological inhibitor zVAD (Figure, right side). Further protein analysis and single cell RNA sequencing in our organoids supported pyroptosis as the specific cell death mechanism leading to endothelial network loss. Multiomic analyses in patients with COVID-19, proteinuric kidney disease, and collapsing glomerulopathy similarly demonstrated increased IFN signaling and pyroptosis-associated gene expression correlated with accelerated kidney disease progression. These results show that IFN-γ signaling itself acutely induces kidney endothelial injury and predisposes cells for pyroptosis, suggesting a synergistic mechanism for APOL1-mediated collapsing glomerulopathy, which might be treatable pharmacologically.
Author Information
Benjamin A. Juliar, Ian B. Stanaway, Fumika Sano, Hongxia Fu, Kelly D. Smith, Shreeram Akilesh, Suzie J. Scales, Jamal El Saghir, Pavan K. Bhatraju, Esther Liu, Johnson Yang, Jennie Lin, Sean Eddy, Matthias Kretzler, Ying Zheng, Jonathan Himmelfarb, Jennifer L. Harder, Benjamin S. Freedman.
Publication
Cell Reports
(2024); 43(6)